Intégrateur
LallianSe [laljɑ̃s] Nom. Intégrateur en sciences de la vie.
Intégrateur [ɛ̃teɡʁa.tœʁ] Nom. Construire l'environnement favorable au succès des innovations.
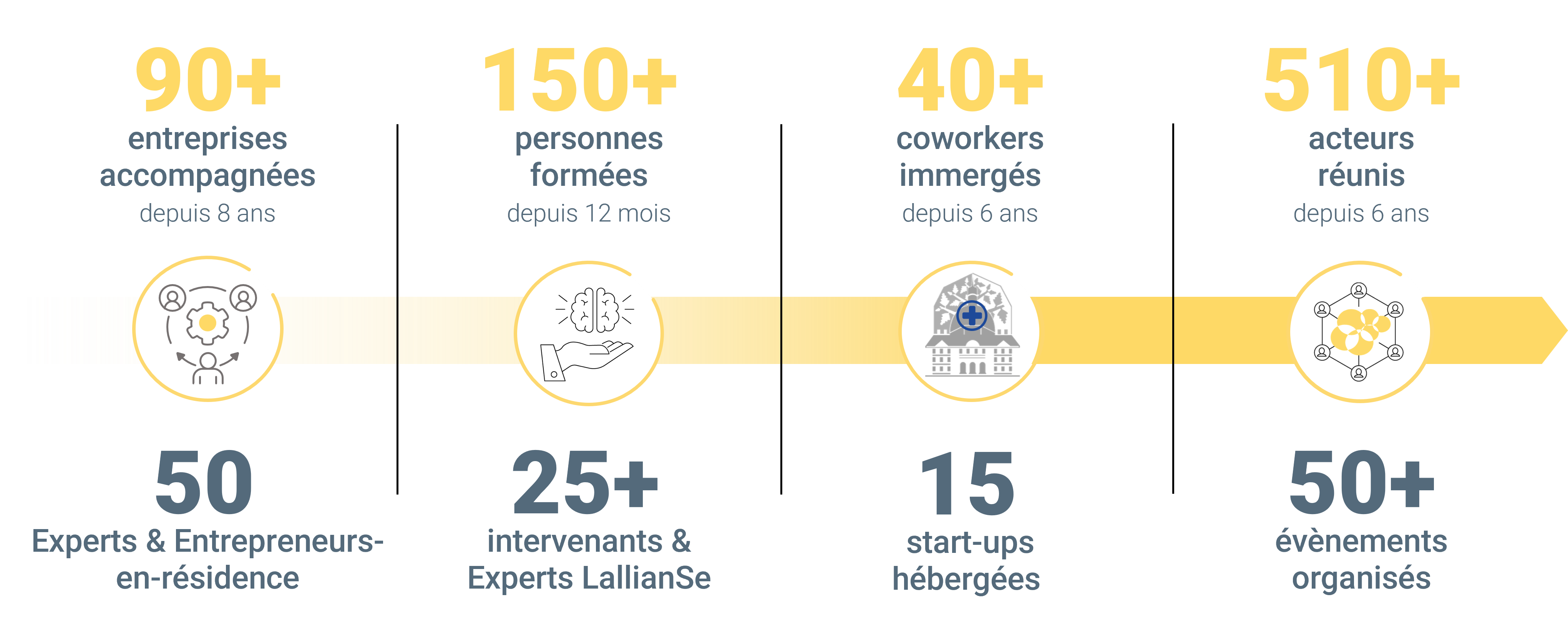
Fondée en 2015, LallianSe est un intégrateur d’innovation qui a déjà accompagné plus de 90 entreprises.
Notre mission : construire l’environnement favorable au succès économique des innovations de santé en articulant des thèses d’investissement.
Nous proposons des parcours sur-mesure pour les institutions, entreprises, hôpitaux et entrepreneurs, avec une approche active, agile et une équipe de 50 Experts et Entrepreneurs-en-résidence.
Notre implantation unique au cœur de l’hôpital nous permet de nouer des relations privilégiées avec les communautés médicale et hospitalière, de former et de fédérer un écosystème sans équivalent au sein du monde de la santé.
Découvrez notre intégrateur 360°
Accompagner
Experts et Entrepreneurs-en-résidence : aux côtés des dirigeants à tous les stades de développement de l’entreprise, de la stratégie à la mise en œuvre.
Éducation
Des programmes de formations à destination des acteurs de l’innovation en santé par des Experts LallianSe, pour partager nos expertises.
Immersion hospitalière
En immersion : espace de co-working au cœur de l’hôpital de la Pitié-Salpêtrière, favorisant l’émergence de relations privilégiées et d’initiatives dédiées.
Évènements
Mensuels et annuels : pour permettre de rassembler, fédérer des partenaires hospitaliers et industriels, entrepreneurs, investisseurs et bien d’autres.
